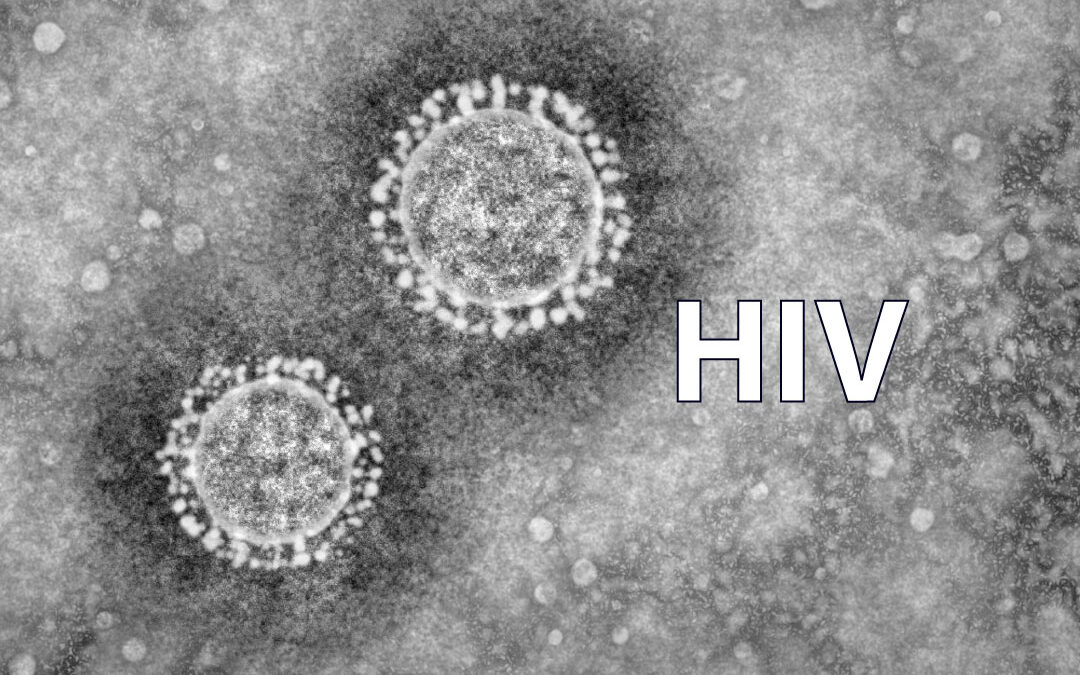
Human immunodeficiency viruses (HIVs) result from cross-species transmissions of simian immunodeficiency viruses (SIVs) that infect African primates. One such event thought to have occurred just after the turn of the 20th century gave rise to the HIV-1 group M, which became the principal cause of the acquired immunodeficiency syndrome (AIDS) pandemic.
AIDS was recognized as a new disease in 1981 and has infected more than 80 million people. It is estimated that 38 million worldwide live with HIV, with around 1.5 million new infections reported yearly. HIV infection can be caused by bodily fluids from an infected person getting into the bloodstream of an uninfected person.
People who are infected with HIV experience a flu-like illness 2 to 6 weeks after infection. Once the symptoms dissipate, usually within 1–2 weeks, there may be no further symptoms for years despite the virus causing inflammation and immune dysregulation. This chronic infection damages the immune system, ultimately leading to AIDS and the inability of the host’s immune system to fight off normal infections.
Classification and Viral Life Cycle
HIV is a member of the Retroviridae family, which uses RNA as its genetic material, reverse transcribes it into DNA, and inserts a DNA copy into the host cell genome. Retroviruses are named after the viral enzyme that copies their genetic material from RNA to DNA, the reverse transcriptase. HIV is also a member of a subgroup of retroviruses named Lentivirus. Lentiviruses are distinguished from other retroviruses because they can infect actively dividing and non-dividing cells, whereas most retroviruses only infect dividing cells.
The virus life cycle can be broken down into separate sections:
Reverse transcription. Upon HIV infecting a cell, the virus uses its reverse transcriptase enzyme to convert its viral genetic material from RNA into DNA, thus making it compatible with the host genome. Reverse transcription has a very high mutation rate, thus generating many new variations and selective advantages.
Integration. Inside the host cell’s nucleus, the virus uses its integrase enzyme to permanently insert a copy of the viral genome into the host’s DNA. Assuming the host cell survives the infection, the viral DNA will remain integrated for the life of that cell. The integration means the virus can continue to produce viral proteins from the cell it has infected or, in the case of some immune cells, can remain in a replication-suppressed status termed ‘latent infection’, where the cells go into a resting state. This silent virus is termed the ‘viral reservoir’, which can be activated later. Therefore, HIV’sintegration is how it can achieve a chronic persistent infection.
Assembly and release. The new viral RNA is packaged with HIV proteins at the inside surface of the cell. The new immature HIV particle forms by budding away from the cell, taking a portion of the host’s cell membrane with it; hence HIV is an enveloped virus. The new virions are released from the cell by hijacking host proteins specialised for this role. The HIV particle then matures and becomes infectious using its protease enzyme to cleave viral structural polyproteins.
Attachment. Once free from the host cell, the infectious HIV particles can bind to cells expressing an immune cell protein CD4 on their surface. Interestingly, despite the huge genetic variety of viruses in an infected individual (arising from the high mutation rate), transmission from person to person is thought to occur via a single virus strain.
Fusion. For HIV to infect a cell, it must fuse its membrane with the host cells. This requires using a co-receptor which is typically another protein expressed on the surface of immune cells – a chemokine receptor called CCR5.
Antiretroviral drugs (ARVs)
Highly active antiretroviral therapy (HAART) is a combination treatment comprised of three or more ARVs. HIVs high mutation rate rapidly generates drug-resistant versions, so the key to the success of HAART is combining drugs that inhibit viral replication by targeting multiple points of the viral lifecycle.
There are six main classes of ARVs that target different stages in the viral lifecycle: nucleoside/nucleotide reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, protease inhibitors, integrase strand transfer inhibitors, fusion inhibitors, and chemokine receptor antagonists.
The sooner HAART is administered after infection, the better the clinical outcome. This is because the swift application of HAART can prevent immune cell dysfunction and the seeding of viral reservoirs. HAART reduces HIV RNA levels, HIV-associated mortality and morbidity, and transmission to nearly zero and has been very successful in preventing mother-to-child transmission. ARVs have recently been used as preexposure prophylaxis (PreP) in high-risk groups, reducing the chance of transmission.
Unfortunately, long-lived infected cells persist during ART HIV DNA. Reactivating the dormant virus is the reason for the resurgence of infection once ART is stopped. Therefore, lifelong treatment is required. The longer the period that viral load is not controlled, the more drug-resistant HIV variants accumulate, which can be transmitted.
There is a constant need for new HIV ARVs to minimize drug side effects and combat HIVs vast genetic diversity and drug resistance. Some stages of the viral lifecycle have yet to be effectively targeted, for example, interfering with capsid assembly or causing premature capsid disassembly. There is still hope that ARVs, alongside other drugs, can provide a path to eradication: the complete removal of the intact and rebound-competent virus. For example, to reactivate latent HIV-infected cells so the cells can be targeted and eliminated (shock and kill) or to suppress or silence HIV transcription (block and lock).
Vaccines
HIV kills and dysregulates cells that are of critical importance to the proper functioning of the immune system. This, combined with an exceptional diversity of sequence within and across the clades, as well as key epitopes on the envelope important for proper virus function hidden within the molecule and protected by an ever-changing glycan shield, means that the immune system usually fails to generate an effective antibody and/or immune response. Although some people can control their viral loads without ARVs, no one is known to have cleared an HIV infection without intervention, and the mechanism(s) or correlates of protective immunity remain unclear, hindering the rational development of a HIV vaccine. There have also been promising developments in the efforts to generate broadly neutralising antibodies that can target many virus variations.
At VRS, we can test the antiviral properties of compounds and antibodies against model HIV-1 strains. If your research requires testing of antiviral molecules against HIV, contact us to see how we can help!
Blog by Scott Lawrence
Edited by Reckon Better Scientific Editing