Antibodies generated in response to flavivirus infections are notoriously cross-reactive, presenting a major obstacle to the accurate differentiation of Zika virus (ZIKV) and dengue virus (DENV). The ability to accurately detect ZIKV infection against a background of endemic DENV infections is crucial to ongoing public health and research efforts worldwide. Poor assay specificity threatens the integrity of clinical assessment and quality of scientific data. Distinguishing the two infections is also critically important for patient management and outcomes, especially in pregnancy.
So what causes cross-reactivity? The Zika virus shares a close relationship with the dengue virus. Both are flaviviruses, transmitted by the Aedes aegypti mosquito and contain ~11kb of positive-stranded RNA that encodes three structural and seven non-structural proteins. Of particular interest to diagnostics and vaccine research are the E (envelope) and NS1 (Non-Structural 1) proteins. The flavivirus envelope glycoprotein is responsible for viral entry to host cells and has been identified as a key target for the development of neutralising antibodies and vaccines [1]. Cells infected with a flavivirus secrete NS1, which plays an important role in immune evasion and pathogenesis and is an early marker of viral replication. Chua et al. (2017) noted that a significant degree of sequence homology has been demonstrated between ZIKV and other flaviviruses, and that a study comparing ZIKV and DENV E proteins indicated up to 53.9% sequence homology [2]. As a result, there are high levels of antibody cross-reactivity between flavivirus proteins when using serological assays.
Overcoming Zika-Dengue Cross-Reactivity
Multiple highly-specific RT-PCR-based assays for detecting ZIKV RNA are available, but their use is restricted to the short time intervals when viral RNA is detectable in body fluids. These timescales are highly variable from patient to patient and since symptoms (if present) are mild, the day of onset may not be discernible. All current serological IgM assays, yield only presumptive results that require confirmatory testing using PRNT titres against ZIKV, DENV and other flaviviruses to which the person may have been exposed, which can take up to 4 weeks.
General approaches to overcoming cross-reactivity in serological testing have included the investigation of an array of antigens from ZIKV as potentially more specific assay targets, together with antigen mutation aimed at removing cross-reactive epitopes while retaining ZIKV-specific regions. While no consensus has been reached as to the ‘best’ antigen, recent findings suggest that NS1 antigen is more specific for IgG antibodies, while envelope antigen is more specific for IgM. However, conventional ELISAs that use traditional ZIKV antigens are still unable to effectively distinguish the presence of other flavivirus infections [3]. It is also clear that using recombinant proteins expressed in mammalian systems offers an advantage over those expressed in other systems.
Novel ELISA formats for specific detection of ZIKV antibodies
Changes in assay design offer an alternative route for investigation. Classical serological ZIKV ELISAs involve measuring the binding of IgM or IgG antibodies present in patient serum to target antigens in a microwell. Since it is these antibodies that often cross-react with other flaviviruses, various immunoassay formats have been tested in attempts to circumvent cross-reactivity, but each have their drawbacks. One approach is to coat anti-IgM on to the ELISA plate to capture IgM antibodies in patient serum, then measure their reactivity with ZIKV. However, such assays tend to be both complicated and lengthy. Others include adding a ‘blocking’ DENV antigen to ‘absorb out’ cross-reactivity, as well as the recently-described ‘Blockade of Binding’ (BoB) assay [4]. None of these options have yet been developed commercially and, while there have been some improvements in commercially available assays, specificity claims are not always borne out of independent studies [5].
Conventional serology ELISAs use an anti-human IgG/IgM antibody conjugated with an enzyme or tag that produces a detectable and quantifiable signal upon binding to the antigen immobilized in the well. Unlike these conventional ELISA formats, double antigen bridging assays (DABA) use an antigen sandwich, with the second part of the sandwich being an antigen conjugated to an enzyme to visualise specific detection.
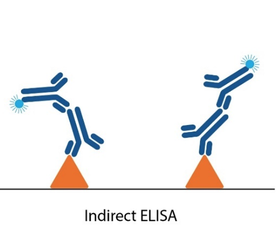
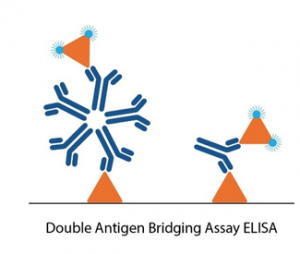
This novel approach to immunoassay design poses several advantages to conventional ELISA formats:
- The DABA assay shows very high specificity, with minimal cross-reactivity to DENV infection. DABA assays also detect higher avidity antibodies, resulting in reduced binding of cross-reactive antibodies.
- DABAs are total antibody assays, meaning that they allow for the detection of all immunoglobulin isotypes and classes – maximising sensitivity.
- DABA formats are not species-specific, making them ideal candidates for immunological studies in animals.
The Native Antigen Company have used the DABA immunoassay format in a new Zika total antibody ELISA kit, designed for immunosurveillance applications, showing extremely high specificity in areas of endemic DENV infection, where cross-reactivity has been a significant problem with earlier assays.
To read more on Zika-Dengue cross-reactivity and immunoassay development, click here for our eBook.
References:
- https://www.researchgate.net/publication/318496441_Antibody-based_assay_discriminates_Zika_virus_infection_from_other_flaviviruses
- https://doi.org/10.1371/journal.pntd.0005269
- https://www.pnas.org/content/114/31/8384
- https://www.researchgate.net/publication/318496441_Antibody-based_assay_discriminates_Zika_virus_infection_from_other_flaviviruses
- https://www.ncbi.nlm.nih.gov/pubmed/29305550




