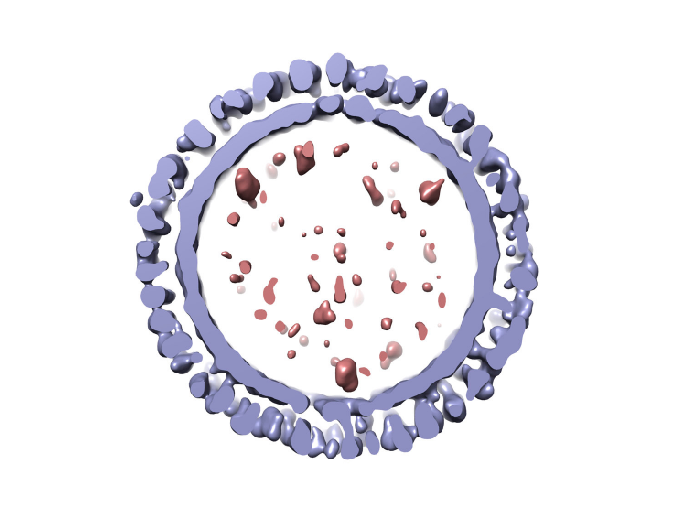
Virus-like particles: life as a shell
Virus-like particles (VLPs) are non-infectious nanostructures made of viral structural proteins, and thereby mimicking the outer conformation of the virus of interest. VLPs might resemble a working virus, but don’t be fooled: these are empty shells that lack the viral genetic material needed to replicate and are therefore harmless. Sometimes an engineered version of the viral genome lacking the genes encoding for the structural proteins can be inserted in the VLPs to allow for expression of a reporter or for gene transfer into the target cells; however, due to the lack of structural components, this genome cannot be incorporated back into new viral particles and spread like the parental virus. Because of their inability to spread or replicate, VLPs do not require the same level of biosafety containment as a replicating virus. Due to their less complex genetic background, VLPs are also much easier to manipulate making them an ideal system for a number of applications including vaccine studies.
VLPs as a vaccine strategy
An important characteristic of VLPs is their ability to self-assemble. VLPs can generate ordered arrays of polypeptides that come together to form the resulting repetitive geometry characteristic of the real virus. By mimicking the virus structure, VLPs can be very useful from a vaccine perspective, as they can stimulate strong humoral and cellular immune responses. These harmless virus mimics are generally more immunogenic than recombinant viral proteins and also of inactivated viruses, when the inactivation process compromises the conformation and immunogenicity of viral antigens. Some VLP vaccines have already been licensed and commercialized: the prophylactic human vaccines against hepatitis B virus (HBV) and human papillomavirus (HPV) – both based on VLPs derived from these viruses – are FDA-approved and are currently in use.
VLPs as delivery systems
The use of VLP to deliver genes or reporters into cells has been widely used in cell biology for many years and it is not surprising that VLPs have also attracted attention as drug delivery systems or for use in gene therapy. This makes sense given that viruses are potent natural delivery vehicles of viral genome into cells in a very selective (thanks to their receptors) and effective (thanks to their ability to avoid certain cellular defences) manner, so why not hijack this ability to develop bespoke delivery systems for small molecules, proteins/peptides, and genes? However, the same reason VLPs work well as vaccines can be a disadvantage for drug delivery as the immune response they induce might hinder their effectiveness as delivery vehicles.
A neat delivery tool
VLPs can be built from the proteins of a single virus or by combining proteins from multiple viruses, thus forming chimeric VLPs.
Some VLPs have a natural tropism to certain tissue, which derives from the parental virus. For example, as hepatitis B virus (HBV) VLP can be used to target hepatocytes and rotavirus VLP could be a specific gut-delivery vector. In most cases, however, VLPs have a rather large natural tropism (like an affinity to sialic acids or heparan sulfates, molecules found on many types of cells and tissues) and such tropism should be restrained. Greater targeting specificity is usually obtained by attaching receptor-recognizing domains to the drug carriers. These targeting domains can be chemically attached to the surface of VLP by conjugation, or the sequence of the domain can be genetically inserted and then expressed in the external domain of the VLP components.
VLPs producing systems
A major advantage of VLPs is that they can be produced in various expression systems. Depending on the complexity of the VLPs, they can be obtained in prokaryotic or eukaryotic cells from recombinant vectors: bacteria, yeast, insect cells, mammalian cells, and plants are all currently used as expression systems. Although leveraging bacteria to produce VLPs is the most time- and cost-effective approach, bacteria cannot perform the post-translational modifications necessary for optimal immunogenicity in humans, compromising their suitability for vaccine production. Also, bacteria might introduce contaminating endotoxins into vaccine formulations. For vaccine production therefore mammalian cells are often the system of choice because they have the capacity to produce complex and accurate post-translational modifications and proper membrane protein folding and assembly. Amongst several mammalian cells, the Chinese Hamster Ovary cell line has been extensively used since it is not human-derived and therefore presents a lower risk of contamination by human viruses. Hek293 cell is another widely used mammalian production platform, which has already been used for the generation of many different types of VLP, such as rabies, HIV, and influenza.
Insect cells are also widely used, particularly for arthropod transmitted viruses. Unfortunately, not all VLPs are produced to the same high titres. VLP systems generated from viruses that bud from the plasma membrane, like retroviruses or alphaviruses, generally yield higher concentrations that systems mimicking viruses budding from internal membrane (e.g. the ER), like the flaviviruses. This can be problematic for large scale vaccine manufacturing, but the yield are generally sufficient for preclinical proof of concept studies.
The power of flexibility
For most viruses all is needed to generate a VLP is the matrix protein giving the budding virus its shape (like HIV Gag, or Ebola virus VP40), and the envelope protein/s. For flaviviruses, which bud from the ER, the envelope protein prM and E are sufficient to generate VLPs.
This gives the system the higher degree of flexibility. Not only VLPs made using one virus system (like the lentivirus system) can be pseudotyped to expose the envelope protein of other viruses, and not only can they be engineered to incorporate genes and reporters, but they can also be used as a valuable expression platform to study the impact of mutations on envelope proteins, without the need to engineer the entire virus, a much more complex task further complicated by genome instability and biosafety restrictions. Also, while every lab generally works with one or possibly few strains of a given virus (often lab adapted), VLPs allow the flexibility of express the surface protein of circulating strains and to study their impact of immunity, or on the ability of an antibody to bind to a wider array of viral strains. This is even more valuable in the context of high-containment and emerging viruses, to which very few lab have access. Harnessing the power of VLPs is very likely to bring us closer to effective vaccine solutions, and to a deeper understanding of how viruses deal with the outside world.