From concept to reality: How a virus is helping to advance gene therapy
This month the FDA approved Zolgensma (Novartis), the first gene therapy for spinal muscular atrophy in children. While the treatment price – $2.125 million – is making most headlines, here we take a moment to highlight the virus that makes this therapy possible.
Adeno-associated virus vector-based gene therapy
The newly approved strategy against spinal muscular atrophy from Novartis is an adeno-associated virus vector-based gene therapy. In its simplest terms, this means that a genetically modified version of a virus (an adeno-associated virus) is used to deliver a functional copy of the survival motor neuron (SMN) gene into the target neurons of affected children. But to frame this story properly, it’s necessary to go back more than 50 years to the discovery of the adeno-associated virus.
Adenoviruses
All the way back in 1953, Rowe and colleagues identified adenoviruses by carefully observing cultured cells from surgically removed adenoids. Something was causing progressive structural changes (reminiscent of a cytopathic effect) in these cells. This ‘adenoid-degenerating (AD) agent’ (Rowe et al. 1953) was soon identified as an adenovirus (Elders 1956), a group of viruses that typically cause respiratory illnesses (e.g., the common cold).
Adeno-associated virus
Later, in 1965, Bob Atchison discovered small “virus-like” particles as contaminants of laboratory adenovirus stocks, which he tentatively named adeno-associated virus (AAV). These particles were antigenically distinct from any known adenovirus antigen, and their replication in cell cultures was obtained only when they were inoculated simultaneously with adenoviruses.
It’s a Dependo-virus because it’s dependent on co-infection helpers
Because a co-infecting helper virus (such as adenovirus or herpes simplex virus) or induction of cellular stress is usually required for a productive infection to occur, AAV serotypes are ascribed to a separate genus in the Parvoviridae family, designated Dependovirus.
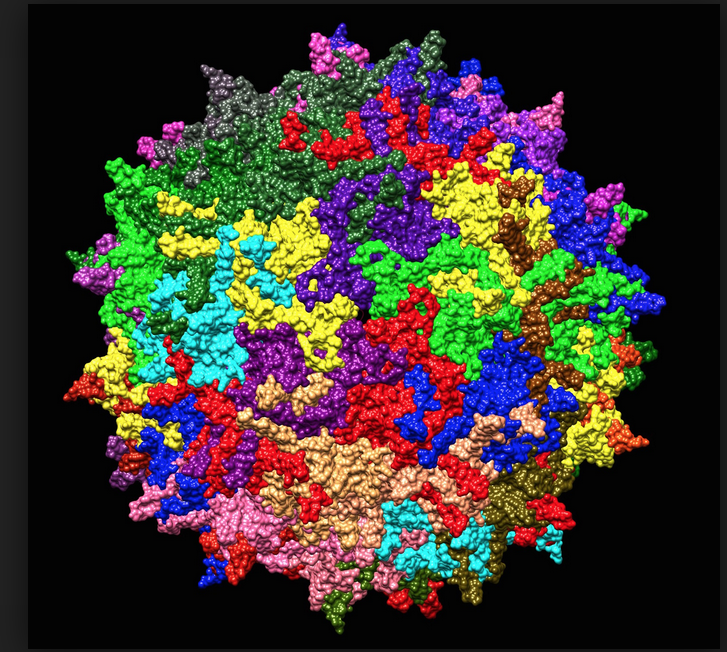
Adeno-associated virus (AAV): Some key facts
AAV has a linear single-stranded DNA (ssDNA) genome of approximately 4.8 kilobases, with two 145 nucleotide-long inverted terminal repeats (ITRs) at the termini, and their symmetry is required for multiplication of the AAV genome. The ITRs are multipalindromic, and they form a T-shaped hairpin that serves as the origin of viral DNA replication (essentially a primer for the initiation of viral DNA replication). Between the two flanking ITRs, there are three viral genes – Rep (Replication), Cap (Capsid), and Aap (Assembly). The rep gene encodes four regulatory proteins that are involved in AAV genome replication, while the cap gene gives rise to three capsid proteins that form the outer capsid shell. The aap gene encodes the assembly-activating protein (AAP) in an alternate reading frame overlapping the cap gene, and it is thought to provide a scaffolding function for capsid assembly. The virus does not encode a polymerase and, therefore, relies on cellular polymerases for genome replication. AAV serotype 2 (AAV2) attachment is primarily mediated by ubiquitous heparan sulphate proteoglycans, which explains, at least in part, the well-known broad tropism of this virus.
The biphasic life cycle of the AAV Dependo-virus
AAV has evolved a biphasic life cycle to ensure persistence in its host:
- in the presence of an unrelated helper virus, such as adenovirus or herpes simplex virus, AAV establishes a productive infection (lytic cycle).
- in the absence of a helper virus, the AAV gene expression program is auto-repressed, and latency is established by preferential integration into a specific site on human chromosome 19, designated AAVS1 (lysogenic cycle). This site-specific integration is orchestrated by the presence of a Rep binding element in the human genome. Site-specific integration of AAV is unique among eukaryotic viruses, and its exploitation holds considerable promise for the targeted integration and stable expression of therapeutic DNA.
- When a latently infected cell is infected with a helper virus, the AAV gene expression program is activated, leading to the AAV excision from the host cell chromosome, followed by replication and packaging of the viral genome. Upon helper virus-induced cell lysis, the newly assembled virions are released.
AAV and its potential as a gene delivery tool
Now, having thoroughly introduced AAV, we come to its potential as a tool, both in research and clinical applications. Multiple key properties distinguish AAV as a gene delivery tool. First, as we’ve described, AAV is a replication-incompetent virus (AAV cannot enter the lytic cycle without help), and it is not known to cause disease in humans. AAV also has a low immunogenic profile, meaning it doesn’t seem to induce much of an immune response. Another useful asset is that AAV can infect a wide range of cell types, both dividing and non-dividing. And an increasing number of AAV serotypes are being identified, which differ in tissue tropisms. In general, the tropism is associated with the abundance of the specific receptor, but there are exceptions, suggesting that there are likely other internalization pathways independent of known receptors. Although multiple serotypes of AAV have been identified, the majority of vectors currently being used are based on AAV2, which is the most studied and best characterized.
Recombinant AAV technologies
Improved knowledge of gene delivery vectors – principally AAV – is now driving a resurgence in gene therapy efforts. AAV is especially suited to various therapeutic applications because of its ability to generate recombinant particles lacking any viral genes and containing DNA sequences of interest.
Typically, a recombinant AAV genome is composed of the transgene of interest, its promoter, and the polyadenylation (poly-a) signal, flanked by the 145 nucleotide-long AAV ITR elements, which are involved in genome amplification and packaging. In many cases, strong, constitutively active promoters are desired to achieve abundant expression of the gene of interest. The region(s) involved in genome replication and virion assembly are provided in trans in a separate construct expressing the genes required for replication and the capsid proteins. Because AAV depends on co-infection with a partner virus to supply essential helper functions for a productive viral cycle, an adenoviral helper plasmid is also needed to induce the lytic life cycle AAV and its release.
The adenovirus helper factors could be provided by either adenovirus infection or by transfection of a third plasmid that provides the helper genes isolated from adenovirus. This latter option gives researchers the ability to produce more virus in a controlled setting, as it would be otherwise very challenging to eliminate the adenovirus from AAV product, and such a contamination is highly undesirable in terms of vector safety and specificity, and unacceptable for clinical purposes.
Long-term transgene expression via AAV
Although recombinant AAV does not integrate into the host genome (because ITRs and rep are not together), transgene expression can be long-lived. In fact, AAV can establish long-term transgene expression. Let’s see how.
AAV-derived vectors deliver a single-stranded DNA genome, which must be converted into double-stranded DNA (dsDNA) by the host cell. Because the virus depends on the cell’s DNA replication machinery to synthesize the complementary strand, transgene expression may be delayed. This process is normally carried out by using the ITR regions. Although AAV can integrate into the host genome in a specific site on chromosome 19, this site-specific integration requires the viral protein Rep, which is normally absent in current AAV vectors. In the absence of Rep proteins, ITR-flanked transgenes encoded within recombinant AAV can form circular concatemers that persist as episomes (high molecular weight structures that are maintained extra-chromosomally) in the nucleus of transduced cells. Therefore, common AAV vectors usually remain within the target tissues as episomal entities. The episomal stability of the AAV vector chromatin enables high level, long-term transgene expression in non-dividing cells. However, since the recombinant episomal DNA does not integrate into host genomes, it will eventually be diluted in dividing cells over repeated rounds of replication.
AAV and the future of gene therapy
AAV is a versatile viral vector technology that can be engineered to suit a variety of clinical applications and to address a range of diseases. This month’s approval of Zolgensma, the treatment of spinal muscular atrophy, is a marker of the advances being made in this field and the important role of engineered AAV in gene therapy. And expect to hear more about AAV in the future: Regenxbio is constructing a facility to produce adeno-associated viral vectors for its gene therapy programs; Pfizer and uniQure are testing AAV-based treatments of Hemophilia B; GenSight Biologics have reported positive clinical findings for their AAV-based treatment of Leber Hereditary Optic Neuropathy – an inherited disease that causes loss of vision; and Spark Therapeutics are having success using AAV to treat Hemophilia A. Exciting times for gene therapy and the viral vector technology that is enabling these advances!