A Quick Refresher: The Hemagglutination (HA) Assay and Its Role in Influenza Virus Detection
Before we delve into the Hemagglutination Inhibition (HAI) assay, let’s briefly revisit the hemagglutination (HA) assay introduced in our previous blog.
The hemagglutination assay (HA assay) is a simple, quick, and cost-effective method for estimating the amount of influenza virus in a sample. It leverages the influenza virus’s hemagglutinin (HA) protein, which causes red blood cells to agglutinate or stick together. The degree of agglutination is used to estimate the virus concentration in the sample.
To perform the HA assay, scientists serially dilute the virus sample in a 96-well plate and mix each diluted sample with red blood cells, typically from chickens or turkeys. The presence or absence of the virus affects the red blood cells’ behavior in the wells. In the absence of the virus, red blood cells settle at the bottom, forming a small red dot. In contrast, when virus particles are present, they bind to the red blood cells, forming a diffuse network that prevents the cells from settling.
Like this, the HA assay provides a rapid visual indicator of the amount of virus in the sample by observing the pattern of red blood cells.
The HAI Assay: Assessing Antibodies and Immunity Against Influenza Virus
The hemagglutination inhibition (HAI) assay, which we’ll discuss in this blog, is a variation of the HA assay. While the HA assay focuses on detecting and measuring the influenza virus, the HAI assay is designed to assess the presence and concentration of antibodies that can neutralize the virus in serum samples. Essentially, the HAI assay measures the immune system’s ability to block the virus’s HA protein, thus inhibiting hemagglutination and providing an estimate of the level of immunity against the influenza virus.
Basic Steps of the HAI Assay
Now that we have an understanding of the HAI assay’s purpose and its connection to the HA assay, let’s dive into the key steps involved in performing the HAI assay to measure the concentration of neutralizing antibodies in serum samples:
- The serum samples are treated with a receptor-destroying enzyme (RDE) to remove any non-specific inhibitors that could interfere with the assay.
- The influenza virus is diluted in a buffer to a predetermined concentration, typically determined by measuring the virus’s hemagglutination titer.
- A dilution series of the serum to be tested is mixed with a fixed amount of virus in every well of a 96-well plate. The mixture of serum and virus is incubated for a short period to allow the antibodies in the serum to bind to the viral hemagglutinin protein.
- A suspension of red blood cells is added to the mixture of serum and virus to detect any hemagglutination caused by the virus.
- The plates are examined for hemagglutination, and the highest dilution of serum that inhibits hemagglutination is recorded as the HAI titer. The titer is reported as the reciprocal of the highest dilution of serum that completely inhibits hemagglutination.
For example, an HAI titer of 64 means that the serum sample has a concentration of antibodies that can neutralize the influenza virus at a dilution of 1:64. The higher the HAI titer, the greater the concentration of neutralizing antibodies in the serum sample.
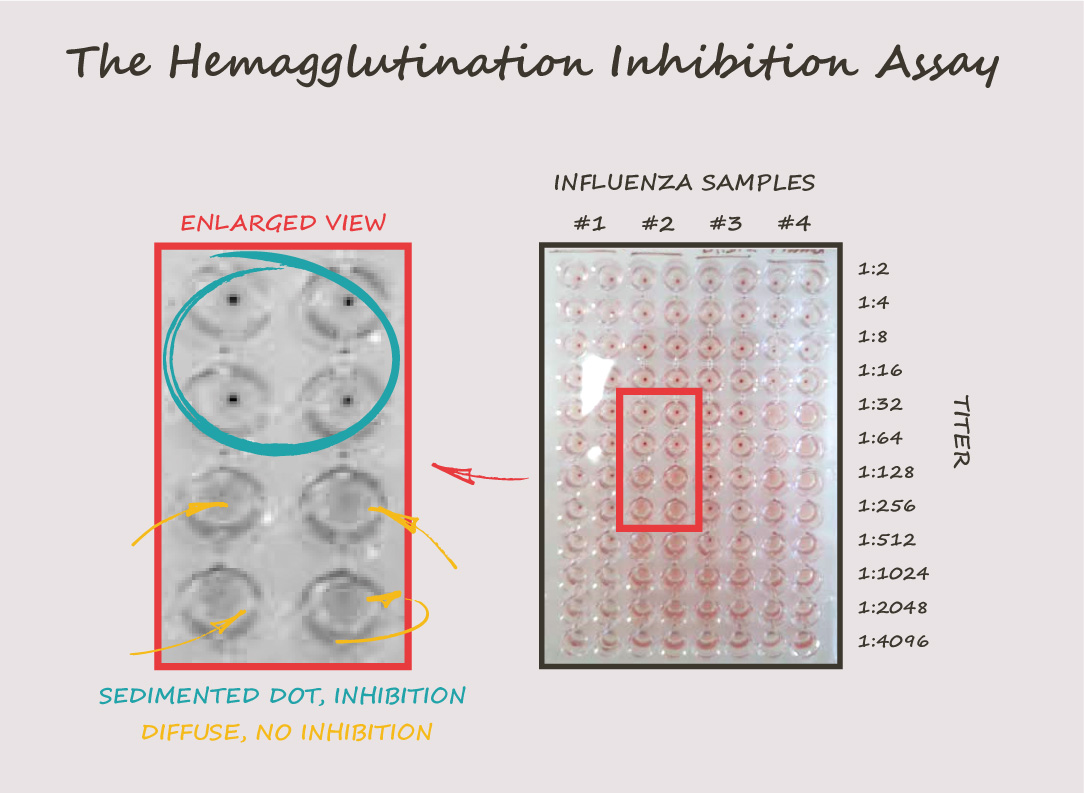
Determining the HA Titer: Laying the Groundwork for the HAI Assay
Before conducting the hemagglutination inhibition (HAI) assay, it is crucial to first determine the hemagglutination (HA) titer of the influenza virus of interest (see our previous blog: HA Assay). This step is essential because it enables researchers to calculate the appropriate virus dilution for use in the assay.
The HA titer measures the concentration of virus particles capable of agglutinating red blood cells in a given sample. In contrast, the HAI assay assesses an antibody’s ability to inhibit red blood cell agglutination by the influenza virus. To accurately gauge this inhibition, a specific quantity of virus particles must be present in the sample. If the virus concentration is too high, it could overwhelm the inhibitory effect of the tested antibody, leading to inaccurate results. Conversely, if the virus concentration is too low, detecting any inhibition of hemagglutination may be challenging.
By first determining the HA titer of the virus, researchers can calculate the appropriate dilution to use in the HAI assay, ensuring accurate and reliable results.
RDE II Treatment: The Bouncer of the HAI Assay
In the hemagglutination inhibition (HAI) assay for influenza virus, treating the serum with the receptor-destroying enzyme (RDE) is a crucial step. This process is essential because it eliminates any non-specific inhibitors in the serum that may interfere with the HAI reaction, potentially leading to inaccurate results.
Sialic acid, a component in serum, can bind to viral surface proteins, including the hemagglutinin (HA) protein, which may prevent the virus from binding to red blood cells and result in false negative outcomes. The RDE enzyme cleaves sialic acid from glycoproteins and glycolipids in the serum, destroying this potential inhibitor and preventing interference with the HAI assay.
By employing RDE treatment, the process removes any potentially troublesome sialic acid-containing molecules not involved in virus neutralization, allowing only the specific antibodies recognizing the HA protein to participate in the assay. This step enhances the accuracy and specificity of the HAI assay and ensures that only the relevant influenza virus-specific antibodies in the serum are measured. Therefore, treating the serum with RDE is a vital step in the HAI assay for obtaining accurate and reliable results.
HAI Assay as a Tool for Monitoring Immune Response to Influenza
The HAI assay is not only used to quantify the level of antibodies to the influenza virus present in serum samples but is also a powerful tool for evaluating immunity to the influenza virus. By measuring the HAI titer, it is possible to assess the immune response’s significance to the virus and the level of protection against infection or disease. This makes the HAI assay an essential tool for monitoring influenza vaccine efficacy and evaluating the effectiveness of public health interventions aimed at reducing the spread of influenza.
Interpreting HAI Titers: The 40 Titer Threshold and Its Implications
An HAI titer of 40 or greater is considered protective against influenza infection. The 40 HAI titer threshold was originally proposed by the World Health Organization (WHO) and is widely used as a correlate of protection for evaluating the efficacy of influenza vaccines. The WHO recommended this threshold based on studies that have shown that individuals with HAI titers of 40 or higher have a lower risk of developing influenza infection than those with lower titers.
However, it is important to note that the HAI titer of 40 as a correlate of protection is not a fixed rule and may vary depending on several factors, including the specific influenza virus subtype, the individual’s age, and the level of pre-existing immunity.
In conclusion
As we continue our fight against influenza, the HAI assay remains a vital tool in understanding our immune defenses, guiding vaccine development, and shaping a healthier future for all.
If you’re interested in HA and HAI assay services, don’t hesitate to contact us. Our team of experts is ready to support your influenza research and vaccine development needs. Get in touch with us today to learn more about how we can help you stay ahead in the fight against the flu.




