For many years, scientists have grappled with the challenge of identifying and describing those things that are invisible to the naked eye. Conventional microscopes, which rely on light properties to visualize samples, have their limitations. Namely, they cannot generate images of subjects smaller than 250 nm (the wavelength of UV light), and most viruses fall below this threshold. Before the advent of advanced techniques like PCR, which can detect viruses through their molecular signatures, scientists had relatively few tools for virus identification.
The invention of the electron microscope
Ernst Ruska and Max Knoll devised the electron microscope in 1931, a different kind of microscope that, instead of using light, transmitted electrons through a specimen to create a two-dimensional (2D) image (transmission electron microscope [TEM]). Advancing his creation, Ruska designed a microscope that deployed a focused beam of electrons to visualize a specimen’s topography, leading to a three-dimensional perspective (scanning electron microscope [SEM]).
These advances began a new era of viewing objects too minuscule for light microscopy, which included the visualization and morphological characterization of tiny infectious agents (viruses) that were previously undetectable.
Throughout the 20th century, electron microscopy was a standard technique for identifying and diagnosing viruses, and it remains indispensable today for identifying obscure emerging viruses.
VRS collaborate with microscopist Steve Gschmeissner
Imaging viruses not only enables us to visualize the cause of our illnesses but it also offers vital insight into viral functions. This can significantly aid the invention and formulation of antiviral agents, vaccines, and, consequently, improved treatments. At VRS, we are deeply interested in viruses, particularly those that infect humans. We investigate a diverse array of viruses, varying in shape, size, and replication method. Some viruses are small and spherical, ranging from 20–200 nm in diameter, while others are tubular, extending into threads exceeding 1 μm. Some viruses have a protein shell and are created internally within the cell, needing the cell to burst for their release. Others are discharged through budding from the cell, incorporating parts of the producer cells’ membranes in the process.
Despite the advances in microscopy, some viruses remain underrepresented in imaging. To address this, we at VRS have partnered with renowned microscopist Steve Gschmeissner (theworldcloseup.com). Gschmeissner specializes in producing and colourizing exceptional SEM images, allowing us to showcase what viruses, such as the one that causes the common cold, actually look like.
Exploring adenovirus
The adenovirus was one of the viruses we decided to image. It typically infects the respiratory system, eyes, and gastrointestinal tract, triggering symptoms similar to the common cold, flu, conjunctivitis, or acute gastroenteritis. Although many 2D images exist of the adenovirus, either independently or within a cell, we managed to capture impressive 3D images of the virus emerging through large openings as the cell lyses – a process unique to its replication and spread. In the following image, you can see the non-enveloped adenovirus particles, which are 70–100 nm in size and coloured in green, emerging from within the cell, shown in blue, through large openings in the cell’s outer membrane.
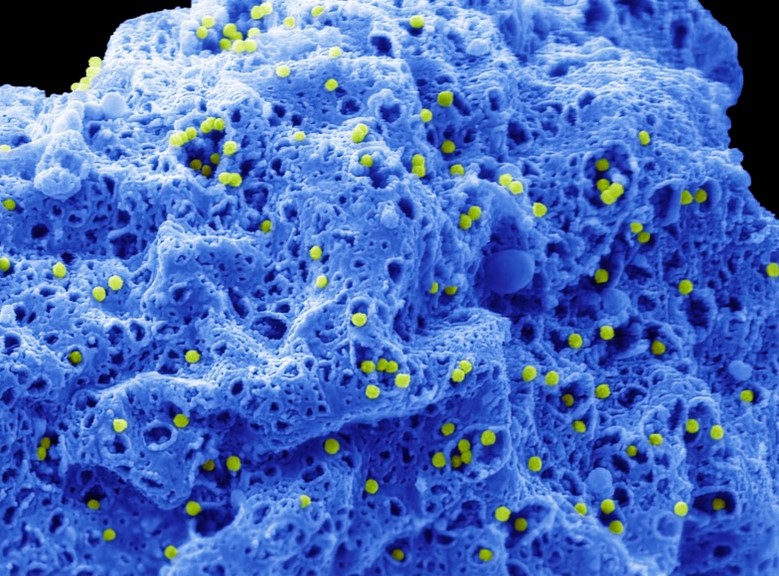
Adenovirus
Imaging respiratory syncytial virus and human metapneumovirus: patterns of cellular emergence
We chose to image two other viruses, respiratory syncytial virus (RSV) and human metapneumovirus (HMPV). Both are common respiratory pathogens typically causing mild symptoms similar to the common cold, such as cough, fever, nasal congestion, and shortness of breath, though they can also lead to more severe conditions like bronchitis or pneumonia.
RSV is recognized for its unique filamentous structure, often several microns long. However, in 2D images, these particles frequently appear to be spherical and pleiomorphic. Examining the imaging, we were surprised to see that in some cells infected by RSV, the production of filamentous virus particles was so high that the original cell shape became unrecognizable. The image below reveals green filamentous RSV (indicated in green) emerging from the cell’s plasma membrane, accompanied by bulging, apoptotic cell surface membrane blebs (shown in pink). This suggests that the underlying cell is undergoing apoptosis or cell death.
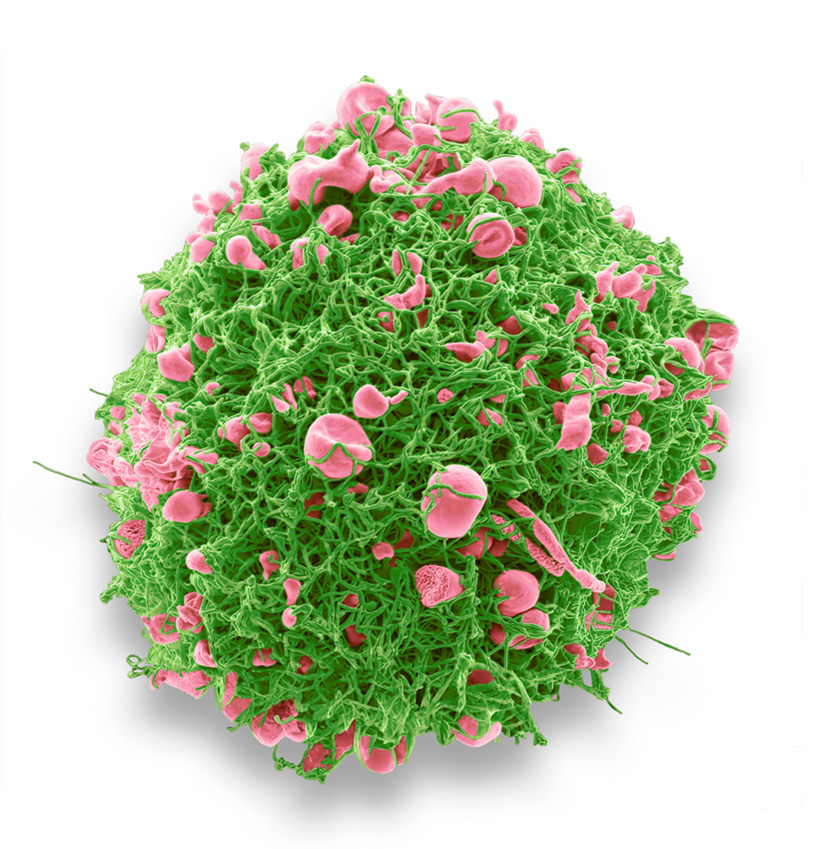
RSV
HMPV was identified only relatively recently, in 2001, leaving us with less knowledge about this virus, which is similar yet distinct from RSV. Interestingly, we observed that both RSV and HMPV can be situated horizontally along the cell membrane or vertically protruding from it. The images below show RSV (left) and HMPV (right), each highlighted in pink.
-
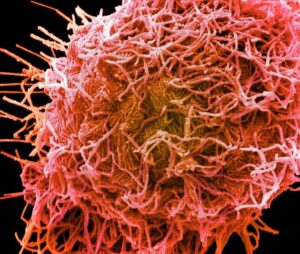
-
RSV
-
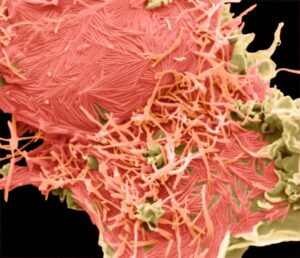
-
HMPV
The significance of viral shape
These images evoke several questions. For instance, why does a virus adopt a long filamentous form rather than a small, round shape? It is speculated that this morphology may aid in the virus’s replication environment and be significant in the cell-to-cell spread of the virus. Also, it is curious why viruses from diverse families tend to bud and emerge from the cell sideways. Ebola research suggests that sideways bursting ensures the complete packaging of the virus’s internal components. Nonetheless, one could also relate this phenomenon to the biomechanics of deforming the plasma membrane to create such a long filament. Despite these theories, we have much more to explore and understand.
Get in touch
At VRS, we’re committed to pushing virus research forward. With advancing technology, we’re excited to uncover new insights into how viruses infect their host cells. Get in touch with our team to learn how we can help overcome the obstacles in your virus research.
Blog by Scott Lawrence