What does a spiral staircase have in common with a tobacco plant virus?
A spiral staircase is an example of helical symmetry, where a shape repeats as it twists and moves along a spiral path. A spiral staircase is efficient because it maximizes space, allowing for vertical movement within a compact footprint.
Nature also makes use of architecturally efficient designs. And with viruses, it’s iconic.
This is the first of a series of blog articles in which we’ll address how viral architectures contribute to their efficacy, and the mechanisms virus particles use to exploit their host.
Virus Structure Terminology
Before we get any deeper, let’s make some notes on virus terminology. You may find it useful to refer back to this list as you read on.
- CAPSID: The protein shell that encloses the nucleic acid.
- NUCLEOCAPSID: The capsid and its enclosed nucleic acid together.
- ENVELOPE: The outer lipid and protein layer that may surround the nucleocapsid, containing material from both the host cell and the virus. The outer structural proteins of some viruses are also often called the envelope.
- VIRION: The complete, fully formed virus particle.
- PROTOMERS: Individual protein subunits that assemble to form the structural units.
- STRUCTURAL UNITS: The smallest functional building blocks of a protein, made up of protomers.
- CAPSOMERS: Visible clusters of structural units on the capsid’s surface.
- FACES: The flat surfaces on the polyhedral capsid, often triangular in icosahedral viruses.
- VERTICES: Points where the edges of the faces meet.
- ICOSAHEDRAL SYMMETRY: When an object’s shape repeats in a pattern with 20 identical triangular faces.
- HELICAL SYMMETRY: When an object’s shape repeats as it twists and moves along a spiral path.

Legend: A Dymaxion globe, a type of world map projection created by architect Buckminster Fuller. It unfolds into an icosahedron. This exemplifies icosahedral symmetry with its 20 triangular faces forming a spherical shape. A spiral staircase, a classic representation of helical symmetry. Each step rotates around a central axis.
The Capsid
Viruses depend on specialized cellular machinery to carry and replicate their genetic material.
The virus capsid is the protein shell that encloses and protects the virus nucleic acids. The capsid not only safeguards the genetic material from external threats but also facilitates its delivery into host cells.
The complexity of the capsid is dictated by the virus’s own genetic material. The more complex the genome, the more intricate the protein shell must be. Viruses have evolved to create symmetrical shells made of repeating protein units to balance size and complexity, minimizing their genetic burden.
Capsids generally fall into two shapes: helical and icosahedral. Helical capsids form elongated tubular structures, like those found in Ebola and influenza A viruses. Icosahedral capsids, on the other hand, are 20-sided polyhedrons, as seen in herpes viruses.
Helical Capsids
Helical capsids are made up of identical repeating protomers that form a protective layer around the virus’s genetic material. These protomers spiral around an axis, creating a hollow cylinder. During replication, the protomers unwind, exposing the genetic material. This helical structure allows for indefinite extension with minimal genetic coding, accommodating large amounts of nucleic acid. Both animal and plant viruses can have helical capsids, with most plant viruses adopting this morphology.
The tobacco mosaic virus capsid: Helical
The tobacco mosaic virus is one of the most extensively studied viruses, primarily affecting tobacco and other plants in the nightshade family. Known for its rod-shaped, rigid structure, tobacco mosaic virus was the first virus to be discovered and is a model organism for studying viral assembly and infection mechanisms. Tobacco mosaic virus’s genetic material is RNA, which is encapsulated within a protective protein coat, the capsid.
The discovery of tobacco mosaic virus marked a significant milestone in virology. Identified in the late 19th century, tobacco mosaic virus was instrumental in proving the existence of viruses as distinct infectious agents. It was the first virus to be crystallized, allowing scientists to determine its structure in detail.
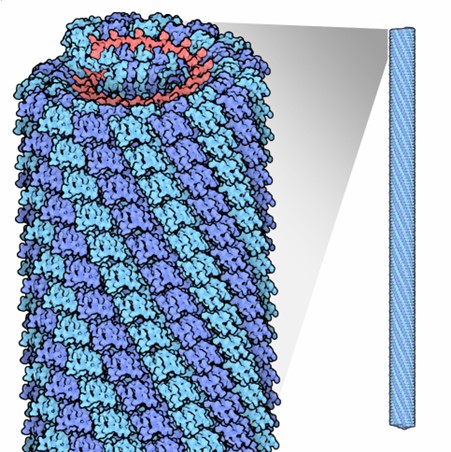
The tobacco mosaic virus has a helical structure. The virus rod is made up of individual protein units called protomers. These protomers assemble into a helix with a repeat length of 6.9 nanometers, consisting of 49 subunits every three turns. Each turn of the helix has about 16 and one-third subunits, resulting in a pitch (the distance between turns) of 2.3 nanometers.
In total, approximately 2,130 protomers cover and protect the virus RNA. The entire virus is 300 nanometers long and 18 nanometers in diameter, with a hollow cylindrical core that is 4 nanometers wide.
This image shows the helical structure of the Tobacco Mosaic Virus. The capsid is formed by protein subunits called protomers, which assemble into a helical configuration around the viral RNA, creating a rigid rod-like shape.
Icosahedral Capsids
Icosahedral capsids, more common than helical capsids, consist of 20 triangular faces. Their formation requires less free energy, making them evolutionarily favored. Unlike helical capsids, icosahedral viruses completely enclose their genetic material within the protein shell, which can house any type of nucleic acid.
However, these capsids also face the challenge of balancing complexity and capacity. Viruses solve this by using repeating structural units. Each triangular face is formed from at least three protein subunits, which can be identical or different. The number of units per face, known as the triangulation number, allows viruses to increase capsid size without additional proteins. Higher triangulation numbers enable larger capsids with few novel proteins.
The arrangement of these protein units is crucial for both structural integrity and host recognition. The interactions between proteins form specialized architectures that aid in host cell recognition and immune evasion.
The adenovirus capsid: Icosahedral
The adenovirus is a well-studied virus that infects humans and animals, causing illnesses from respiratory infections to gastroenteritis. Known for its icosahedral structure, the adenovirus’s double-stranded DNA is encased in a robust protein coat, making it a model for studying viral infection mechanisms.
Discovered in the 1950s, adenoviruses are important in molecular biology and gene therapy. They efficiently deliver genetic material to host cells, offering insights into viral replication and immune evasion, and aiding in vaccine and therapy development.
The adenovirus capsid has 20 triangular faces, each with 12 hexon trimers, totaling 240 hexon trimers. These major capsid proteins provide structural integrity. At each of the 12 vertices are penton capsomeres, consisting of a penton base and a fiber protein, crucial for host cell attachment.
The capsid measures about 90 nanometers in diameter, with fiber proteins extending another 30 nanometers. This precise assembly of hexon and penton proteins makes the adenovirus a key subject in virology research.
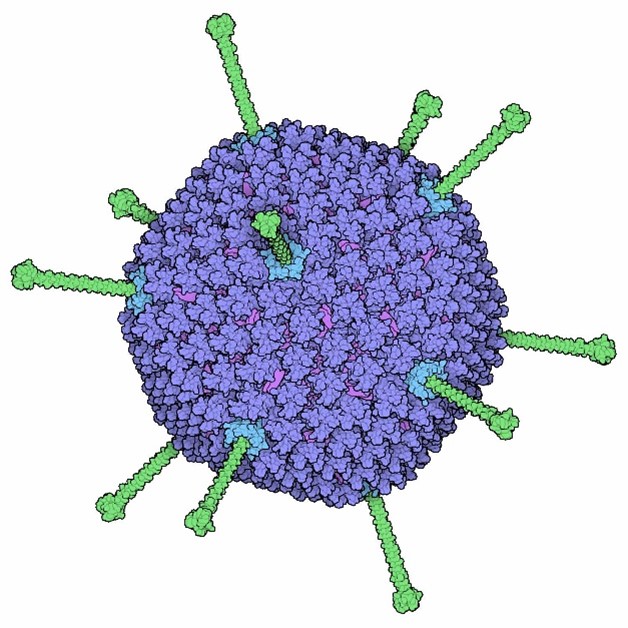
Adenovirus, with 240 hexon trimers (blue) forming its faces and penton capsomeres (green) at each of the 12 vertices. The fibers extending from the vertices are key for host cell attachment and entry.
A Deeper Look at the Capsid
Capsid protein subunits are held together by electrostatic and hydrophobic forces. The organization of these subunits is key to the capsid’s ability to self-assemble, guided by specific genome interactions. These interactions are controlled by packaging signals on the genomic material, ensuring precise assembly.
Despite its importance, the molecular mechanisms of capsid packaging and host interactions remain poorly understood. Unravelling these processes could reveal new drug targets.
Next in the Series: Enveloped vs. Non-enveloped Viruses
The capsid is just one part of a virus’s architecture. Stay tuned for our next post, where we’ll explore the differences between enveloped and non-enveloped viruses and their structural components.
Need Assistance?
Do you have a promising antiviral drug candidate or library you’d like to screen? Virology Research Services can help advance your virus research project. Contact us today, and one of our senior scientists will get back to you.
Blog by Caroline Chapman
Edited by Reckon Better – Scientific Communication Services