New season, new challenges. As the influenza landscape evolves each season, researchers face the ongoing challenge of staying current with emerging viral variants. VRS is pleased to announce the availability of the 2024-2025 WHO-recommended influenza vaccine strains, ready to support antiviral testing and neutralization assays.
2024-2025 Influenza Strains: Available Now for Your Research
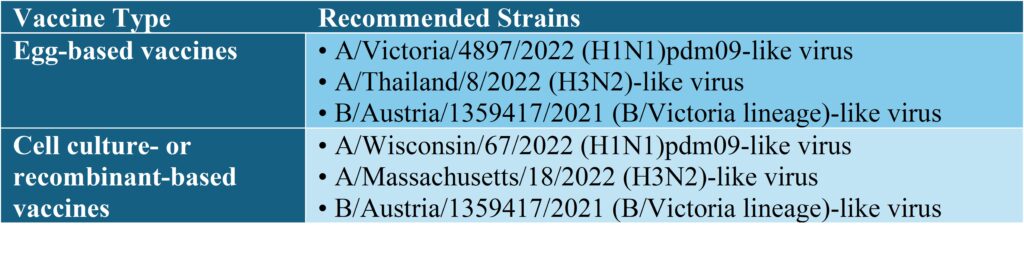
VRS work with the following WHO-recommended influenza vaccine strains for the 2024–2025 season:
H3N2 Influenza
A/Thailand/8/2022
A/Croatia/10136RV/2023
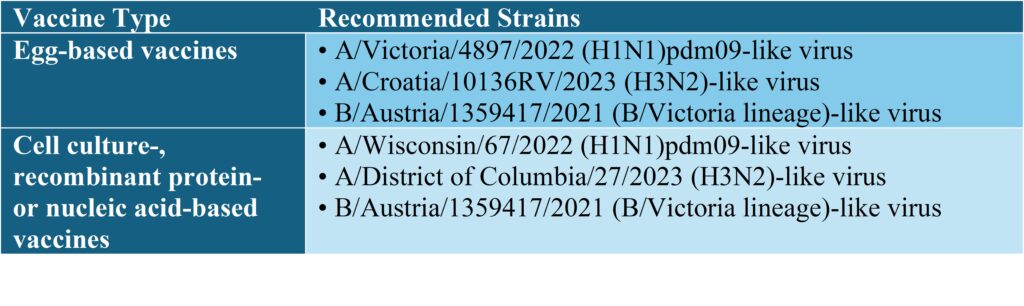
A/District of Columbia/27/2023
H1N1 Influenza
A/Wisconsin/67/2022
A/Victoria/4897/2022
Influenza B/Austria/1359417/2021 (Victoria lineage)
How Influenza Vaccine Strains Are Selected
Combating the continuously evolving influenza virus requires annual vaccine updates to target emerging strains with the potential to cause widespread infection or severe disease.
The World Health Organization (WHO) coordinates a sophisticated global surveillance network that monitors circulating influenza viruses year-round. This extensive data collection enables WHO experts to convene twice annually – in February for Northern Hemisphere recommendations and September for Southern Hemisphere formulations – to analyze trends and identify the most likely prevalent strains for upcoming seasons.
Following these expert consultations, vaccine manufacturers develop, test, and distribute formulations based on WHO recommendations, ensuring healthcare providers receive updated vaccines before the onset of flu season.
2024-2025 WHO Recommendations
Northern Hemisphere (2024-2025)
Southern Hemisphere (2025)
Important 2024-2025 Updates for Influenza Researchers
Transition from quadrivalent to trivalent vaccines
A significant development for the 2024-2025 influenza season is the recommended removal of the B/Yamagata lineage component from seasonal vaccines. While still included in quadrivalent formulations as B/Phuket/3073/2013-like virus, this lineage has not been detected in circulation since the onset of the COVID-19 pandemic.
Following WHO guidance, the European Medicines Agency (EMA) has recommended transitioning from quadrivalent to trivalent vaccines, which now exclude the B/Yamagata component. This shift represents an important evolution in seasonal influenza vaccination strategy and has implications for research protocols and clinical development programs.
Understanding annual vaccine composition changes
Influenza viruses evolve continuously, though not always at a pace requiring complete reformulation of vaccines each year. The WHO’s decision to update specific strains is based on several factors:
- Antigenic drift assessment: When circulating strains show minimal genetic or antigenic changes, existing formulations may be maintained
- Prevalence patterns: Strains causing the majority of infections often remain in the vaccine composition
- Production considerations: Changes to vaccine strains require extensive testing, manufacturing adjustments, and regulatory approvals
These deliberate decisions ensure vaccines target the most likely circulating strains while maintaining production efficiency and reliability.
Influenza Vaccine Effectiveness Trends
Historical data from the past 15 years reveals significant variability in influenza vaccine effectiveness, typically ranging between 19% and 60% according to CDC statistics:
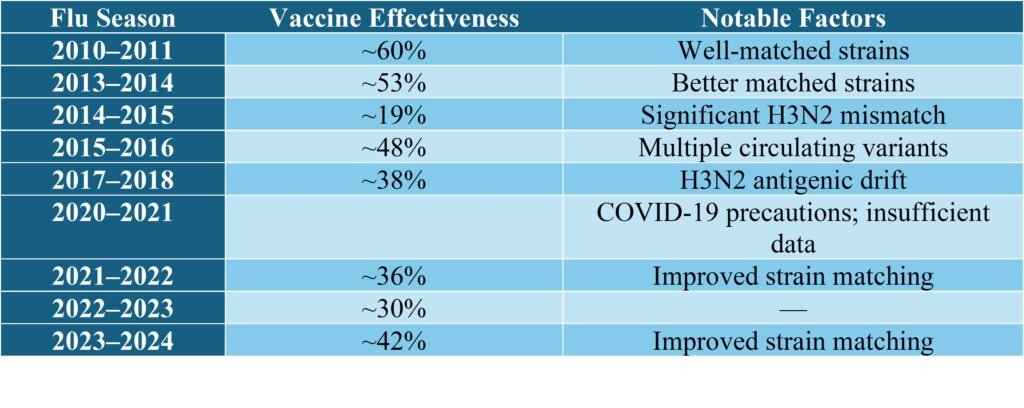
This variability underscores the importance of research to enhance vaccine effectiveness against evolving influenza strains.
Advanced Research Directions in Influenza
The quest for universal influenza vaccines
The development of a universal influenza vaccine remains one of the field’s most compelling research objectives. Unlike current seasonal vaccines requiring annual updates, a universal vaccine would provide long-term protection against diverse influenza strains, including those with pandemic potential.
The scientific approach focuses on targeting conserved viral regions that undergo minimal mutation, including:
- The stem region of hemagglutinin (HA) protein
- Internal viral nucleoproteins
- Epitopes generating cross-protective immune responses
Broad-spectrum antibody therapeutics
Beyond vaccines, broad-spectrum antibodies represent an emerging therapeutic approach with significant potential, particularly for immunocompromised patients and those at high risk of complications. These antibodies:
- Target conserved viral regions providing protection across multiple influenza strains
- Can be administered prophylactically or therapeutically
- May reduce the severity and duration of influenza infections
- Complement vaccination strategies, particularly for vulnerable populations
Our Comprehensive Influenza Research Service Portfolio
To address the complex challenges in influenza vaccine and therapeutic development, VRS offers a strategically designed service portfolio that supports your research journey from early discovery through pre-clinical evaluation:
Antiviral & vaccine development support
Our platform supports both traditional and next-generation approaches to influenza prevention and treatment:
- IC50 determination for novel influenza antivirals against seasonal and pandemic strains
- Comprehensive drug screening with customizable panels of influenza subtypes
- Advanced infection models including air-liquid interface cultures and organoid systems that better recapitulate human respiratory physiology
- Neutralization assays with validated protocols for both seasonal and potentially pandemic influenza strains
Precision detection & characterization
Our analytical capabilities provide the critical data needed for development decisions:
- Viral quantification using gold-standard methods including TCID50, plaque assays, and immunofocus techniques
- Sensitive molecular detection through optimized qPCR protocols for influenza identification and subtyping
- Antibody characterization with ELISA-based services and high-throughput imaging of influenza-infected cells
- Cross-reactivity assessment to evaluate antibody performance across influenza subtypes
Specialized applications & industrial testing
Beyond traditional research applications, we support specialized needs:
- Surface and material testing (ISO21702, ISO18184) for companies developing influenza-resistant products
- Disinfectant validation (DIN EN 14476) to confirm efficacy against influenza strains
- Transport device evaluation to ensure sample integrity for diagnostic applications
- Custom protocol development aligned with your specific research questions and regulatory requirements
With VRS as your research partner, you gain access to both cutting-edge capabilities and the expertise needed to navigate the evolving influenza landscape effectively.
Continuous Influenza Strain Updates
At VRS, we frequently update our seasonal influenza strain repertoire and extend our neutralisation assay to each new strain, supporting vaccine and antibody research through a thorough optimisation and verification process for each strain.
Check with us to see if we already work with your strain of interest or if we can acquire it. We’ll be happy to assist with your project.
Next Steps: Schedule Your Consultation
Ready to accelerate your influenza research with VRS’s expert team and the latest strains?
Complete our quick project inquiry form. One of our experienced virologists will contact you quickly to begin a customized study proposal.
We can then get started with rapid onboarding and clear project milestones.




