TL;DR: Streamline your viral titer assays with our free TCID50 Calculator for fast, accurate Reed-Muench or Spearman-Kärber results.
Table of Contents
Every virologist knows the scenario: you’ve completed your infectivity assay scoring and now face the TCID50 calculation step. Whether you’re using established spreadsheet templates or existing online calculators, the process often involves more friction than it should for such a fundamental measurement.
After years of using various calculation methods in our own research, we developed a solution that addresses the workflow inefficiencies we consistently encountered. Our free TCID50 calculator was designed specifically to streamline this critical measurement process. It is now available at: https://vrsxp.com/experiments/tcid50-calculator/
Why TCID50 Accuracy Matters
TCID50 (Tissue Culture Infectious Dose 50) quantifies the viral concentration required to infect 50% of a cell culture population. For virology professionals, precise TCID50 determinations are fundamental to experimental reproducibility and data integrity.
Accurate TCID50 measurements serve as the foundation for determining viral loads, standardizing experimental conditions, and ensuring reliable data for critical research decisions. Yet despite their importance, the calculation process has often remained more cumbersome than necessary, creating bottlenecks in laboratory workflows.
The Current TCID50 Calculation Landscape
TCID50 calculations are typically done using spreadsheet templates and online calculators. However, our experience revealed consistent challenges with these approaches that impact both efficiency and reliability.
Complex spreadsheet dependencies can be problematic when a formula needs to be modified or when troubleshooting calculation errors. More concerning is the potential for silent errors – a single misplaced cell reference, an accidentally modified formula, or a corrupted calculation can introduce systematic errors that may go undetected for months or even years.
When surveying online calculators, we found that many were inadequately maintained. In some cases, undocumented bugs were identified, creating a genuine likelihood that users have reported wrong TCID50 values.
Manual calculations provide complete methodological control, but introduce opportunities for computational errors and can significantly impact laboratory throughput. It may also require more advanced mathematical knowledge than most lab scientists typically possess, especially when greater flexibility is needed, such as working with different dilution series or varying starting concentrations.
These challenges represent genuine workflow weaknesses that can impact research timelines and data quality, issues we believed could be addressed through thoughtful tool design.
Our Solution: Purpose-Built for Modern Laboratory Workflows
We developed the TCID50 Calculator to directly address these workflow challenges while respecting the methodological foundations established by the research community. Our approach focused on several key design principles:
Flexible Calculation Methods
Implementation of both Reed-Muench and Spearman-Kärber methodologies ensures compatibility with established laboratory protocols and facilitates result comparison across research groups. With the TCID50 Calculator, users can select their preferred method based on institutional standards or collaborative requirements.
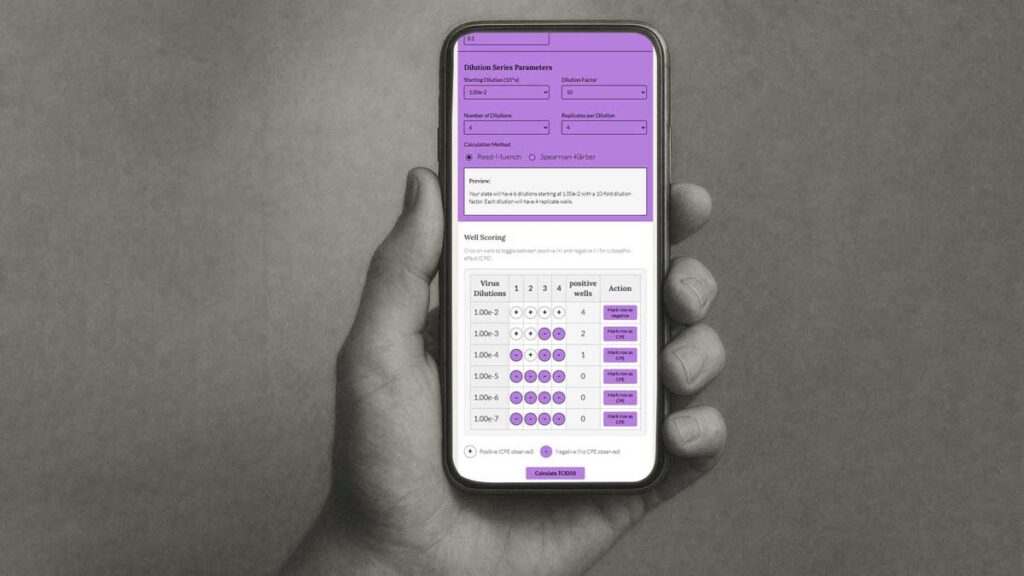
In addition, the tools provide exceptional flexibility, allowing to input different starting concentrations, dilution series, inoculum volumes, and replicate numbers. Want to run a 1.7-fold dilution series? No problem: just enter it using the “Custom” option!
Streamlined Data Entry
Visual plate scoring eliminates transcription errors through direct well selection – CPE-positive wells are marked in purple, and negative wells remain grey. The TCID50 Calculator has an intuitive interface that reduces data entry errors and improves scoring efficiency compared to traditional grid-based approaches.
Comprehensive Documentation
Integrated metadata capture includes sample identifiers, experimental dates, operator information, viral strain details, cell line specifications, and incubation parameters. This documentation is essential for traceability and experimental reproducibility, often requiring separate tracking in conventional approaches.
Immediate Results with Transparency
Real-time calculation processing using the TCID50 Calculator provides immediate results with detailed calculation steps, eliminating processing delays while maintaining complete visibility over the maths and the thinking.
Data Integration Capabilities
CSV export functionality enables seamless integration with laboratory information management systems and electronic lab notebooks without requiring manual data reformatting.
Applications Across Virology Research
This TCID50 calculator addresses workflow requirements across multiple research and development contexts:
- Vaccine development programs that require precise viral load standardization
- Antiviral compound screening, where accurate IC50 determinations depend on reliable viral titer measurements
- Viral stock characterization, ensuring consistent infectivity parameters across experimental batches
- Multi-site collaborative studies requiring standardized methodology and consistent result interpretation
Development Philosophy and Future Direction
VRSxp is our breakout space for creative ideas that can impact virology. Each project or ‘experiment’ is an attempt to find those sweet spots where advances in knowledge and technology allow us to hit an unmet need.
The TCID50 Calculator is our first VRSxp ‘experiment’. More will follow.
Getting Started
The TCID50 calculator is freely available at VRSxp.com with no registration requirements or usage restrictions. We encourage researchers to use it and welcome feedback on additional features that would enhance laboratory productivity.
For questions regarding the TCID50 methodology or broader virology research support, our team at Virology Research Services is available to discuss your specific requirements and research objectives.




