When to use a suspension test
We perform suspension tests to test whether a liquid formulation is virucidal. And if so, the most important thing is that this test also tells us how strong the virucidal activity is.
These tests are especially useful in making choices or recommendations about chemical disinfectants or hand rubs/washes. For example, a suspension test might be used to test how well a hand wash inactivates a virus(es).
For this reason, suspension tests are popular with researchers who develop any liquid formulation intended to inactivate viruses. For example, the R&D department of a chemicals company. It’s often much easier to sell a product with proven virucidal efficacy (more on this later).
Background
The suspension test for testing disinfection procedures dates back to the late 1800s and early 1900s. It was first used to measure bactericidal activity. And since then, the test has been developed in many ways. The modern version is more standardised, reproducible, and quantitative.
Important considerations when planning a suspension test
When designing a suspension test, it’s important to consider how the product will be used in the real world. For example, will the liquid suspension be diluted? What will be the contact time with the virus? Will it be hot or cold? Might there be dirty conditions, such as soil or body secretions? It might also be important to ensure the test aligns with other international standards, such as ASTM-E1052. Therefore, the test should include conditions to simulate where and how the product will be used.
That’s why it’s best to work with an experienced team. Here at VRS, we conduct these types of tests daily and will be glad to help anyone that might need guidance.
Which viruses to test against when using a suspension test?
The test organisms are an important consideration in the design of the assay. Viruses have varied structures. And this gives them a broad spectrum of stability. So, the results against one virus cannot be easily extrapolated to other viruses.
Enveloped and non-enveloped viruses in suspension tests
Whether a virus has a lipid envelope (enveloped) or protein shell (non-enveloped) is an important consideration. Also, can the virus-of-interest dissolve in fats (lipophilic) or water (hydrophilic)? These factors play a part in determining virus stability/persistence.
Non-enveloped viruses typically have greater resistance to inactivation/disruption than enveloped viruses. For example, a small quantity of a non-ionic detergent can render some enveloped viruses non-infectious in seconds. Whereas some non-enveloped viruses are hardly affected after hours of treatment. Thus, it’s prudent to choose a test virus that is as close as possible to the virus the product will target. For a more detailed discussion of these points, we recommend the work of Steinmann (https://pubmed.ncbi.nlm.nih.gov/11759018/) and Tarka and Nitsch-Osuch (https://pubmed.ncbi.nlm.nih.gov/33804814/).
Experimental principles of measuring virucidal activity using a suspension test
Here we describe the basic suspension test and the various controls that are either performed as standard practice or can be added to a suspension test to provide further detail on the product and assay performance.
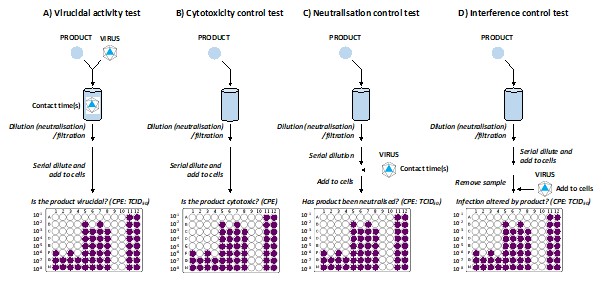
Figure 1. Panels A–D outline the procedure for different parts of a suspension test.
A) Virucidal activity test
This is the main test to discern if a test product is virucidal (see panel A in Figure 1). To perform a suspension test, a virus stock is mixed with a test product liquid formulation. The liquid formulation should be homogeneous and physically stable. The virus and test product are then incubated, usually at room temperature, for the required exposure time (also called contact time). It’s not unusual to test multiple contact times, aiming to identify the shortest time needed to inactivate the virus.
At the end of the product-virus contact time, the test product is neutralised by dilution. It is then time to measure how much viable virus has survived. This is done by adding the neutralised product and virus mixture to host cells and looking for evidence of infection. This is called virus-induced cytopathic effect (CPE).
CPE is a term that covers many things and can be specific to the virus. CPE is usually observed as changes in cell shape (morphology) or cell damage, or death caused by infection of the host cells. The more infectious virus that survives contact with the test product, the more CPE we expect to see.
Using a 96-well plate format and making a serial dilution of the recovered virus, we then score the wells for the presence and absence of CPE. Using this information, we can calculate a titre, usually expressed as the TCID50 (Tissue Culture Infectious Dose, the dilution of a virus required to infect 50% of a given cell culture). For more on TCID50, you can read our blog article Timeless TCID50 (https://virologyresearchservices.com/2019/03/29/timeless-tcid50-one-solution-to-many-viruses/).
Positive and negative controls
Concurrent with the above experiment, two important control experiments are also performed. In one, the test product is replaced with a harmless control buffer that doesn’t inactivate the virus (the negative control). In the other, the test product is replaced with a solution that we know will inactivate or disrupt the virus (positive control).
Using the negative control data
Using the negative control data – where we’ve used a harmless solution – we can test whether the test process is altering the virus. This is something we want to avoid. We do this by performing a parallel dilution of the same stock virus that was used for the test (but has not gone through the process) and comparing how much virus is recovered from the negative control condition to how much virus was added to the experiment to begin with.
Calculating antiviral activity
Next, we can calculate the antiviral activity for the test product. We do this by comparing the amounts of virus recovered from the test condition (product-of-interest) and negative control (harmless buffer). We consider a product antiviral if the virus titre is reduced by at least four logarithmic steps in the product condition (relative to the harmless buffer), taking into consideration the results of the cytotoxicity control (discussed below).
Further details about antiviral suspension tests
Below, we go into detail about some of the additional control experiments we include as standard or can include in our suspension tests for virucidal activity. These additional controls ensure the test data is valid, giving confidence in the result.
B) Cytotoxicity control test
For the virucidal test to be valid, the test product toxicity must not prevent the measurement of viral titre reduction . As we’ve discussed above, the suspension test relies on using host cells to detect infectious virus. Sometimes, the carryover of the test product into the wells containing the host cells can cause toxicity to the cells. Because this toxicity might resemble CPE, this can confuse the test results. So, we must determine if, in the virucidal test, the CPE is caused by viral replication or simply the product damaging the cells. We check for this in every test and do this using the cytotoxicity control test.
To measure cytotoxicity, the same test discussed in A is performed, but without adding the virus (see panel B in Figure 1). Then, the cytotoxicity is quantified by examining any morphological change or cell damage (CPE). Because no virus is added, any cytotoxicity to the host cells must be due to the carryover of the product. With this information, we can distinguish between virus-induced and product-induced effects.
Ideally, the test product will not display CPE after being diluted/neutralised. But, microbicides are often toxic to cells at working concentrations. So, if the dilution or neutralisation step is insufficient (i.e., we cannot measure virus-induced CPE due to cytotoxicity), a filtration step can be included. This filtration is done after the neutralisation step. It reduces the concentration (and hence carryover) of the test product while retaining any recovered surviving virus.
C) Neutralisation control test
A useful control to add to a suspension test is a neutralisation control. The neutralisation control tests whether the neutralisation step has been successful. If the product’s antiviral activity has been successfully neutralised, it will not impact the virus beyond the neutralisation step. If the product’s antiviral activity hasn’t been sufficiently neutralised, it can continue to impact the virus after the neutralisation step. When neutralisation fails, the suspension test data is considered invalid. Passing this neutralisation control is a requirement of the ATSM-E1052 standard.
To test the neutralisation step, we follow the same test described in B (the cytotoxicity control). Then, after the neutralisation step (see panel C in Figure 1) and serially diluting the product, we add the virus. This mixture of virus plus neutralised product is then left to incubate for the same contact time as the corresponding virucidal activity test. After leaving this mix for the defined period, the amount of surviving virus is measured using host cells and the CPE method.
When neutralisation has been successful, we expect to recover as much infectious virus from this neutralisation control test as we do from the negative control condition (harmless media) in the virucidal activity test.
D) Interference control test
Another useful and informative control that can be added to a suspension test is an interference control. This test checks if exposure to product carryover (after neutralisation) alters host cell susceptibility (negatively or positively) to viral infection.
To measure interference, the same test discussed in B is performed up to the neutralisation step (see panel D in Figure 1). After the neutralisation step, the product is serially diluted and added to the host cells.
This neutralised product carryover is incubated with the host cells, then removed. Now a serial dilution of the virus is added to the pre-treated host cells, and the amount of surviving virus is measured using host cells and the CPE method. When neutralisation has been successful, we expect to recover as much infectious virus from this neutralisation control test as we do from the negative control condition (harmless media) in the virucidal activity test. This allows us to see whether transient exposure to the neutralised product alters subsequent cell-virus interaction.
What can VRS do?
VRS can test your solutions against any of our extensive virus library (https://virologyresearchservices.com/viruses/). Contact us (https://virologyresearchservices.com/contact/) with any enquires. We can customise the assay to your requirements, such as contact times and temperature, adding a soil/protein load, and include neutralisation or interference controls to fully assess the performance and virucidal activity of your products. Need to test the antiviral activity of a surface or fabric instead? We can also help with that (https://virologyresearchservices.com/services/industrial-antiviral-testing-services/).
Further Reading
1. Steinmann J. Some principles of virucidal testing. J Hosp Infect 2001; 48 (suppl A): S15-S17. (https://pubmed.ncbi.nlm.nih.gov/11759018/)
2. Tarka P and Nitsch-Osuch A. Evaluating the Virucidal Activity of Disinfectants According to European Union Standards. Viruses. 2021 Apr; 13(4): 534. (https://pubmed.ncbi.nlm.nih.gov/33804814/)
Blog by Scott Lawrence
Edited by Reckon Better Scientific Service




