


The mathematics of qRT-PCR in virology
Virologists spend an awful lot of time counting virus particles, in all sorts of ways, and for many sorts of reasons. But ‘not all that glitters is infectious’, when we’re counting virus particles. In the previous article, we focused on quantifying active viruses –...
3D cell culture: Revolutionizing how we study health and disease
Imagine studying a city from a flat map – you’re missing the depth, the real interactions. Now imagine walking through its streets. That’s the kind of impact 3D cell culture is making for in vitro cell biology. The Origins of 3D Cell Culture For decades, 2D cell...
What do viruses look like?
For many years, scientists have grappled with the challenge of identifying and describing those things that are invisible to the naked eye. Conventional microscopes, which rely on light properties to visualize samples, have their limitations. Namely, they cannot...
CLSI M40-A2 Standard
In the scientific and medical communities, the accuracy of specimen collection and transportation is crucial. For example, if the transport conditions are not adequately maintained, such as incorrect temperature or delays in transportation, it can cause degradation of...
Studying RSV Infection Using the Air-Liquid Interface Model
When studying virus infection (or, in fact, any complex biological phenomenon), it is natural to wonder whether the cell system of choice adequately recapitulates the complexity of an organism. This is also true for respiratory infections, where not only the airway...
Air-liquid interface testing services
Welcome to our blog about air-liquid interface (ALI) testing services. As the demand for more effective and reliable preclinical models continues to grow, the use of ALI technology has become increasingly popular. At Virology Research Services, we provide ALI testing...
Understanding the HAI Assay
Navigating the intricacies of immunity against the influenza virus is no easy task, but the hemagglutination inhibition (HAI) assay has proven to be a powerful asset in this complex puzzle. As a vital tool in virology and immunology research, the HAI assay provides...
The Hemagglutination Assay
Every year, influenza wreaks havoc on our health, but have you ever wondered how scientists detect and measure the amount of flu virus in a sample? The answer lies in a unique property of a key viral protein and an assay called the hemagglutination assay....
How to test if a liquid is antiviral: Suspension test for virucidal activity
When to use a suspension test We perform suspension tests to test whether a liquid formulation is virucidal. And if so, the most important thing is that this test also tells us how strong the virucidal activity is. These tests are especially useful in making choices...
Quantitative PCR validation for research scientists: Part 2 of 2
A two-part primer in quantitative PCR validation This two-part article discusses the validation of quantitative PCR (qPCR) assays and why validation is essential for you as a research scientist to have confidence in your data. This discussion includes clear and...
Quantitative PCR validation for research scientists: Part 1 of 2
A two-part primer in quantitative PCR validation If you’re a research scientist using quantitative PCR (qPCR), you must consider validation. Without validating your qPCR assay, you’ll never have confidence in your data. In this two-part article, we’ll discuss the most...
Resistance passaging
What is resistance passaging? Resistance passaging is a method researchers use to investigate how microbes evolve resistance to drugs. Why is resistance passaging useful? Drug resistance is when a microbe overcomes a drug’s ability to kill it or prevent its...
How do you go about making a vaccine? – II Part
Preclinical testing comes at a relatively early stage in vaccine discovery, as the development of an immune response following antigen administration can only be tested in an organism. If adequate models are available, protection from (or attenuation of) viral...
How do you go about making a vaccine? – I Part
How do you go about making a vaccine? We received this very pertinent question from a sci-fi author a few weeks ago, and thought it was worth a new short blog series! This month we cover the early R&D stages of vaccine discovery, while in the next blog, we’ll talk...
Antiviral drug discovery – Part 3: When the virus fights back – antiviral resistance
In this three-blog series, we present the typical workflow of an antiviral discovery program, from the initial identification of antiviral molecules in vitro (Part 1) to preclinical testing (Part 2) and assessing the onset of drug resistance (Part 3). These early...
Antiviral drug discovery – Part 2: From candidates to investigational drugs
In this three-part blog series, we present the typical workflow of an antiviral discovery program, from the initial identification of antiviral molecules in vitro (Part 1) to preclinical testing (Part 2) and assessing the onset of drug resistance (Part 3). These early...
Antiviral drug discovery – Part 1: From no drug to promising candidates
From target to drug Traditionally, antiviral drug discovery has started from detailed knowledge of a specific viral target. Proteins that are critical for a virus’ life cycle (e.g., polymerase, protease, and envelope) are first characterised, ideally by...
How to look at… Part 3/3: Viral assembly and release
In this series of three blogs, we present different assays and techniques that can be used to narrow down the mode of action of compounds or cellular proteins that interfere with the virus life cycle, and we focus on procedures that – with some optimisation – can be...
How to look at… Part 2/3: Viral replication
Gaining access to the cell is a prerequisite to establishing infection. Replication, however, is when a virus subverts the cell to generate multiple copies of itself. The nature of the virus genome determines the steps required to reach this stage. Positive-stranded...
How to look at … Part 1/3: Viral entry
Understanding at what stage of a virus life cycle a compound or a restriction factor acts is critical in the discovery process. In drug discovery, this aspect can be a bottleneck for hits generated via phenotypic screenings, but even when the viral of cellular targets...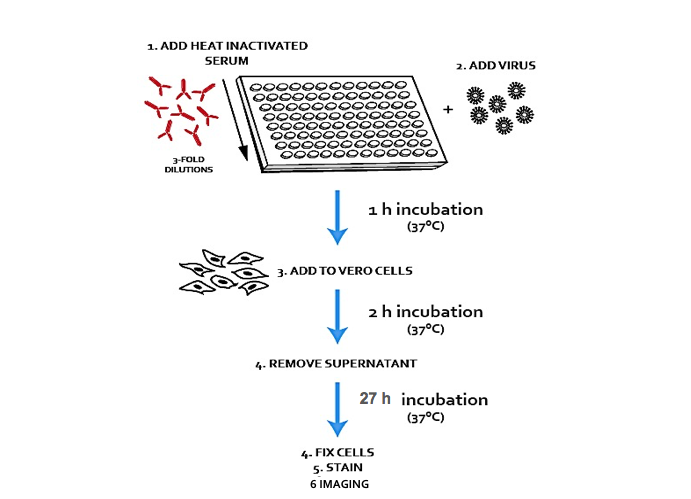
Assay of the month: The microneutralization assay
A milestone in vaccine development is the generation of high concentrations of potent and specific antibodies able to neutralize the virus of interest. Antibodies mostly work by inhibiting viral entry, and as such they have the ability to block infection very early...Page 1 of 1
